Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kitatsuji, Yoshihiro
Radioisotopes, 67(10), p.483 - 493, 2018/10
Electrochemical reactions and redox properties of actinides such as uranium and neptunium are outlined. The flow electrolysis enables rapid and high-efficient treatment. It was demonstrated to measure slow processes of actinide redox. Experimental results of electrolysis of actinide ions and the preparation method of oxidation state of the ions based on the fundamental data are described. Mediator reaction and catalysis observed in the process of electrolysis of actinide ions are also explained.
Aoyagi, Hisao*; Kitatsuji, Yoshihiro; Yoshida, Zenko; Kihara, Sorin*
Analytica Chimica Acta, 538(1-2), p.283 - 289, 2005/05
Times Cited Count:15 Percentile:41.55(Chemistry, Analytical)Redox behavior of Np(III), (IV), (V) and (VI) ions in aqueous perchloric, nitric and sulphuric acid solutions was elucidated by using column electrodes connected in series in a flow system. Using a glassy carbon fiber working electrode as the column electrode was found to be very useful for the investigation of current-potential relations of not only reversible redox processes such as Np(VI)/(V) or Np(IV)/(III) but also irreversible processes such as Np(V)/(IV) or Np(V)/(III), the latter not observed by conventional voltammetry which uses a glassy carbon or platinum electrode. Quantitative electrolysis could be executed even if the redox process was completely irreversible by the use of the column electrodes. By taking advantage of column electrode electrolysis, a novel method was developed for the rapid preparation of a neptunium species of a desired oxidation state, including unstable species. The multi-step column electrode system was demonstrated to be useful for the coulometric determination and speciation of Np(IV), (V) and (VI) in aqueous HNO solution as an example.
solution as an example.
; ; ; ; ; *; *
JNC TN8400 2001-026, 29 Pages, 2001/12
The measurement condition by spectrophotometry was evaluated to measure Np content in MOX fuel containing Np. The Np concentration was obtained by measuring the 727nm absorption peak, after the valence of Np in the sample solution was adjusted to the Np(Ⅳ). The calibration curve showed the linearity up to Np concentration 0.8mg/ml. Though Pu and U quantity were respectively added to the Np solution to 30 times and 60 times of Np concentration, there was no effect to the Np analysis. By using this method, relative standard deviation (RSD) of the analyzed values of Np content for 2%Np - MOX fuel was about 4%. In addition, It was confirmed that the Np content could be measured without separating Np from Pu and U. This method can be sufficiently applied as a quick simple method to analyze Np content in MOX fuel containing Np.
Hirata, Masaru; Tachimori, Shoichi; Sekine, Rika*; Onoe, Jun*; Nakamatsu, Hirohide*
Advances in Quantum Chemistry, Volume 37, p.335 - 351, 2001/00
no abstracts in English
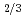 TiO
TiO
Yoshii, Kenji
Journal of Alloys and Compounds, 305(1-2), p.72 - 75, 2000/06
Times Cited Count:13 Percentile:61.56(Chemistry, Physical)no abstracts in English
*; Nakazawa, Toshiyuki*; Ueta, Shinzo*; Shibata, Masahiro
JNC TN8400 99-069, 41 Pages, 1999/11
As a part of the evaluation for the sorption phenomena of nuclides in compacted bentonite, apparent diffusivities for uranium, neptunium and technetium that are redox-sensitive elements, were measured under reducing conditions. Bentonite used was a sodium bentonite, Kunigel V1. Apparent diffusivities were measured by using in-diffusion method (concentration profile method), under the conditions with varying dry densities of compacted bentonite and sorts of the solution used for water saturation of bentonite in diffusion experiments. As a result of the measurements, following ranges of values for apparent diffusivities were acquired. ...
 O
O
Ikeda, Naoshi*; Yamada, Yasusada*; Nodo, S.*; Inami, Toshiya; Katano, Susumu
Physica B; Condensed Matter, 241-243, p.820 - 822, 1998/00
Times Cited Count:21 Percentile:73.59(Physics, Condensed Matter)no abstracts in English
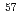 Co using a specially designed cryostat for time differential coincidence emission Moessbauer spectroscopy of
Co using a specially designed cryostat for time differential coincidence emission Moessbauer spectroscopy of  Np
NpSaeki, Masakatsu; Nakada, Masami; ; Yoshida, Zenko; *; *; Yamashita, Toshiyuki; ;
Hyperfine Interactions, 92, p.1177 - 1181, 1994/00
Times Cited Count:7 Percentile:48.79(Physics, Atomic, Molecular & Chemical)no abstracts in English
Sakurai, Satoshi; Usuda, Shigekazu; ; Hirata, Masaru; ; Tachimori, Shoichi
Nihon Genshiryoku Gakkai-Shi, 35(2), p.147 - 154, 1993/02
no abstracts in English
Sasaki, Teikichi
Hyomen, 30(11), p.915 - 927, 1992/00
no abstracts in English
Kudo, Hiroshi
Kagaku To Kogyo, 45(5), p.941 - 944, 1992/00
no abstracts in English
 U
U O
O solid solutions
solid solutionsHinatsu, Yukio
Journal of Solid State Chemistry, 95, p.430 - 437, 1991/00
Times Cited Count:5 Percentile:27.71(Chemistry, Inorganic & Nuclear)no abstracts in English
Kudo, Hiroshi
Kagaku, 46(11), p.748 - 752, 1991/00
no abstracts in English